Clinical, control, reliability
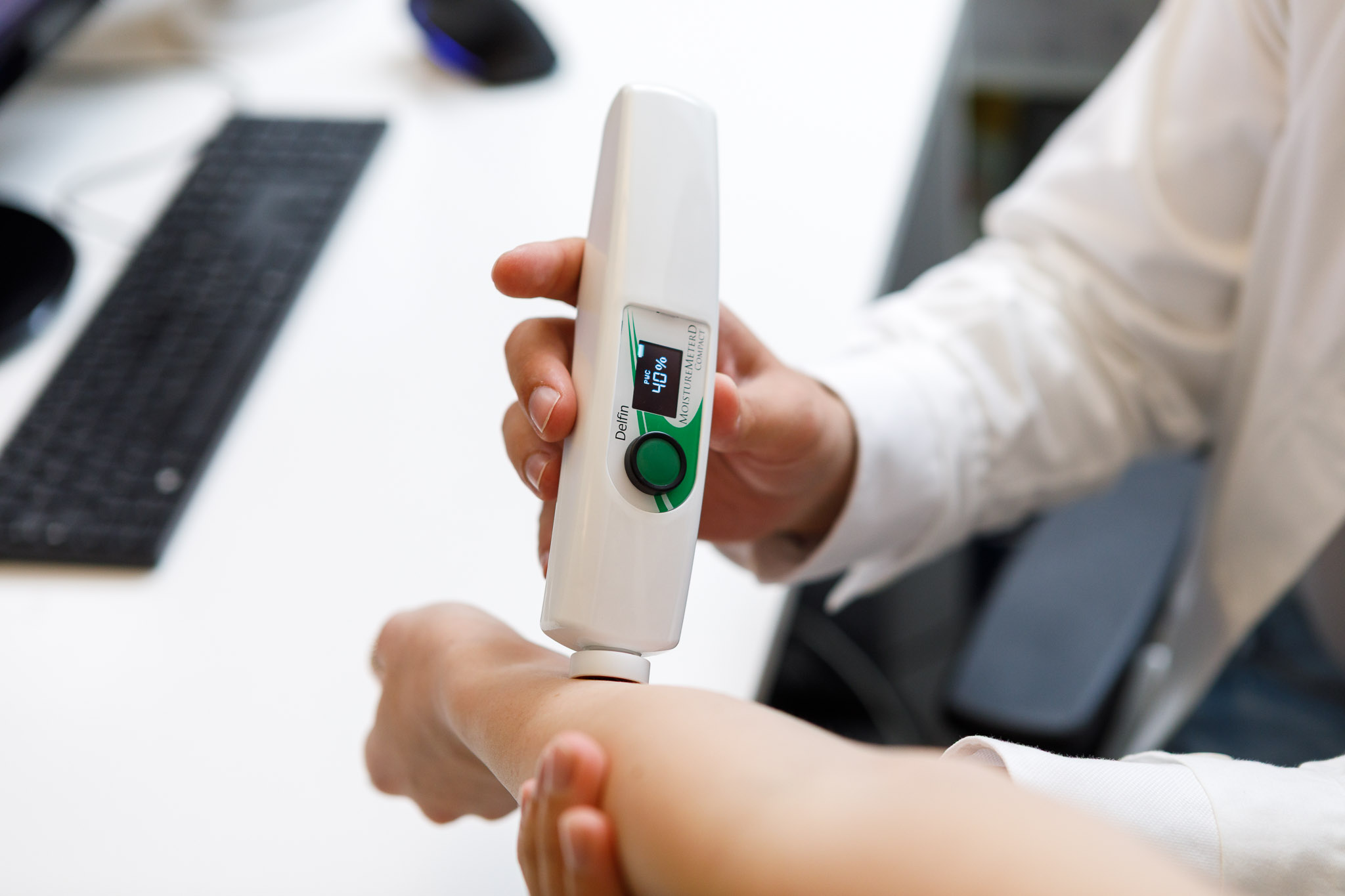
In vivo safety tests are essential to confirm that a product intended for skin contact is well tolerated and safe for use.
Bio Basic conducts dermatological, gynecological, ophthalmological, pediatric, and odontoiatric evaluations on selected volunteers, with the aim of identifying any potential irritation, sensitization, adverse reactions, or other unwanted effects.
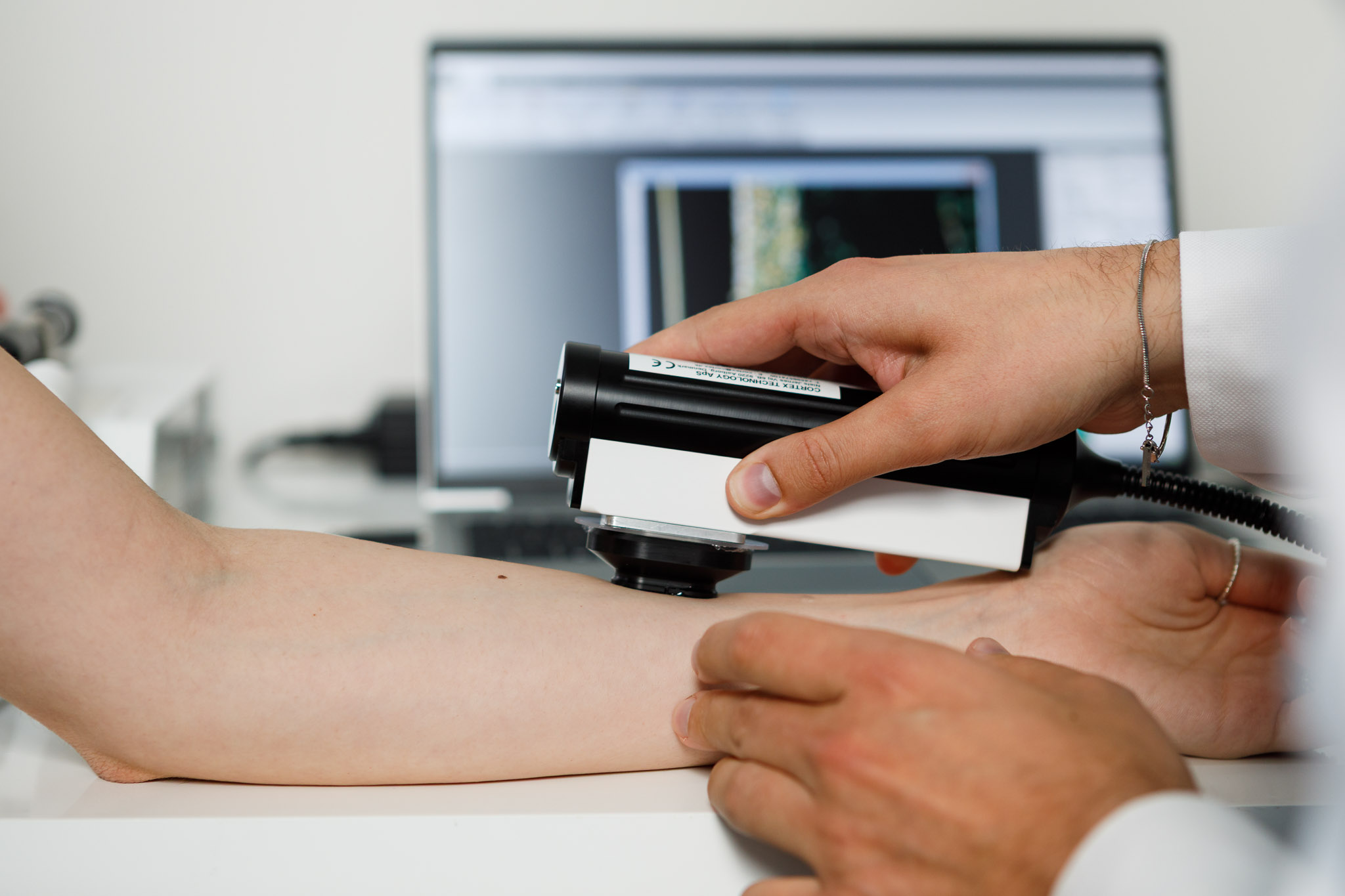
These assessments are carried out under the continuous supervision of medical specialists and through CE-marked non-invasive biomedical devices that allow accurate monitoring of skin responses and the collection of objective clinical data.
Thanks to structured protocols fully aligned with EU and international regulations, Bio Basic ensures reliable, ethical, and scientifically rigorous studies. The well-being of volunteers and the quality of clinical data remain at the core of our work, offering companies the highest level of assurance regarding product safety before market release.